G Ranganathan is the director of Srisan Pharma. His company has made Coldrif cough syrup.
G Ranganathan, director of Shreesan Pharma, the company that manufactures Coldrif cough syrup, which was the cause of death of 23 children in Madhya Pradesh, has been arrested. SIT had announced a reward of Rs 20,000 for anyone giving information about him. Ranganathan’s Chennai in Chennai
,
Meanwhile, a big revelation has come to light in the investigation of Coldrif syrup. The report of Tamil Nadu Director of Drugs Control has revealed that this syrup was prepared from non-pharmaceutical grade chemicals.
During investigation, the owner of the company verbally admitted that he had purchased two bags of 50 kg of propylene glycol on two occasions. That means the company had purchased 100 kg of poisonous chemical. No bill was found during investigation. No purchase entry was made. During interrogation, he told the investigating officers that the payment was sometimes made in cash and sometimes through G-Pay.
Deputy CM and Health Minister Rajendra Shukla reached Nagpur late on Tuesday night and met the sick children admitted in the hospital.
The amount of poisonous chemical is 486 times more

The drug manufacturing company purchased poor quality propylene glycol. He was never even tested. The surprising thing is that the company neither has the purchase bills nor the records of the chemicals used.
Lab testing also found that the presence of toxic chemicals like diethylene glycol (DEG) and ethylene glycol (EG) in the syrup was 486 times more than the prescribed limit.
Here, an expert, on the condition of anonymity, has said that this quantity is not only fatal for a child but it can also destroy the kidneys and brain of an animal the size of an elephant.
Chemical was purchased in March According to the investigation report, the company had purchased propylene glycol from Sunrise Biotech of Chennai on March 25, 2025. But, it was of non-pharmaceutical grade. That means it was not suitable for making medicine. Despite this, the company neither checked its purity nor tested the amount of diethylene glycol or ethylene glycol in it.
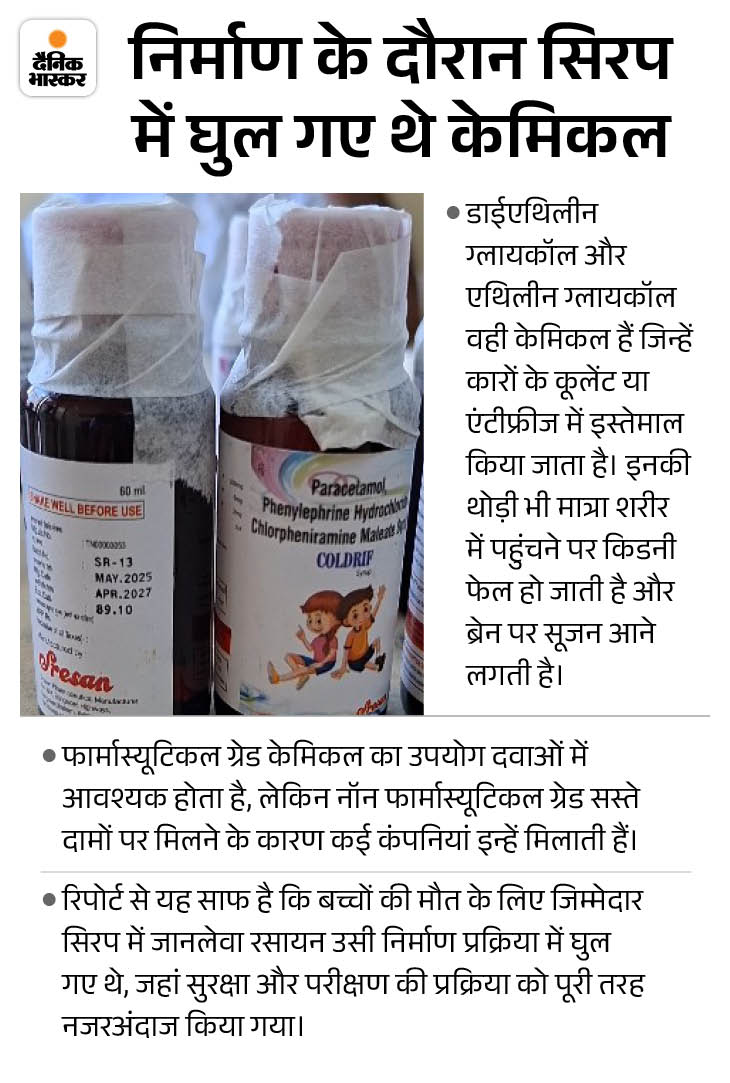
The company tried to hide the documents Tamil Nadu Drugs Control Department found that many medicines were prepared from this substandard chemical. In such a situation, the investigation team continued its investigation during the inspection. In which he found that the company did not have any stock of propylene glycol at that time. This deepened the suspicion that the company tried to hide the documents by quickly disposing of the chemical.
Tamil Nadu Drug Control Authority said that this investigation was extremely necessary in the interest of public safety, as medicines made using non-pharmaceutical grade chemicals can prove fatal for children and adults.

589 bottles of Coldriff were to be sent to Chhindwara. The investigation team had found 589 bottles of Coldrif syrup (batch number SR-13) of 60 ml at Srisan Pharmaceuticals. Which was prepared to be sent to Chhindwara. Drinking the syrup of the same batch number caused kidney failure and swelling in the brain of the children. Which became the cause of his death. These syrups were prepared in the month of May 2025. Whereas, the expiry date is the year 2027 in the month of April.
The investigation team found 5870 bottles of syrup Apart from Coldrif, the investigation team found 4 more syrups from the manufacturing site of the pharmaceutical company. These included 1534 bottles of Respolite D, 2800 bottles of Respolite GL, 736 bottles of Respolite ST, and 800 bottles of Hepsandin syrup. However, during investigation it was found to be of standard quality.

,
Read this news also…
Government admitted that Chhindwara incident was caused by chemical poison: Drug controller removed in MP, three officers suspended

In Chhindwara and Betul, 19 children have died so far due to kidney failure after drinking cough syrup containing toxic elements. In this matter, CM Dr. Mohan Yadav has removed Madhya Pradesh Drug Controller Dinesh Maurya. The CM has also given instructions to suspend Food and Drug Administration Deputy Director Shobhit Koshta, Chhindwara Drug Inspector Gaurav Sharma and Jabalpur Drug Inspector Sharad Jain. Read full news here



