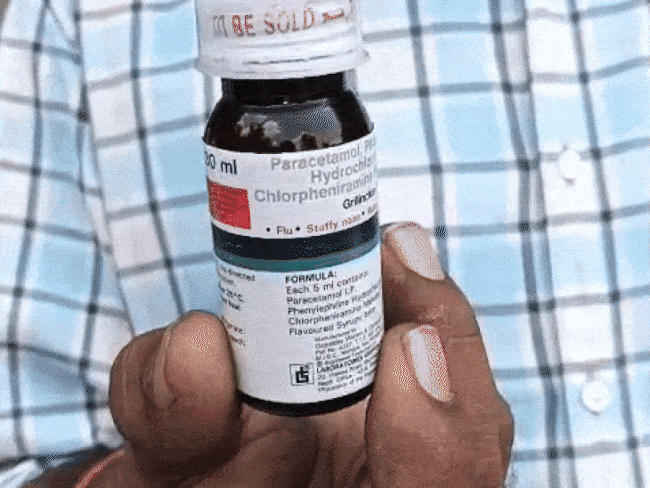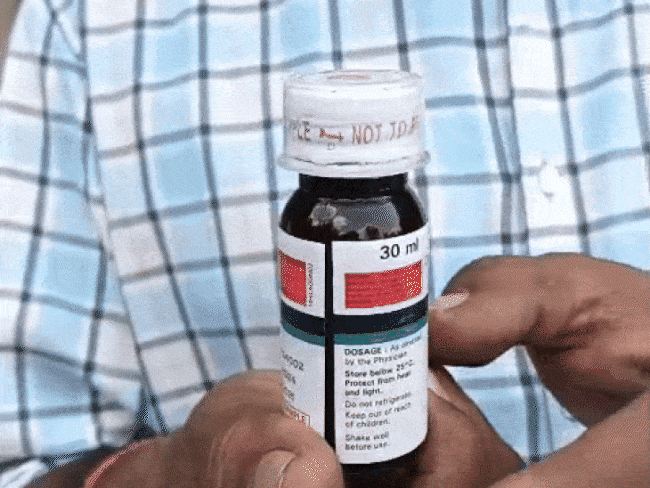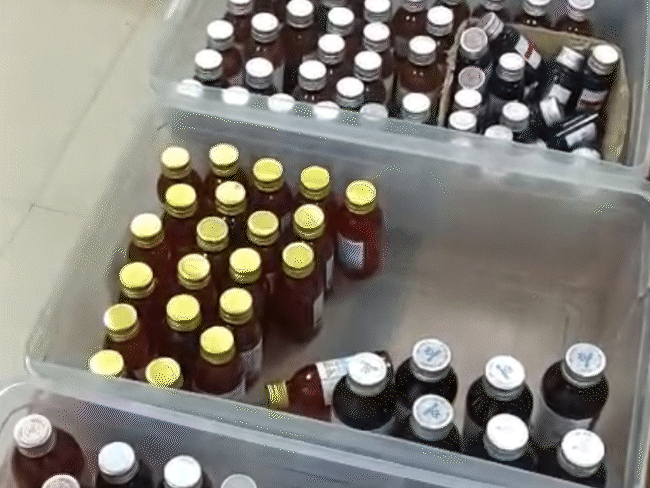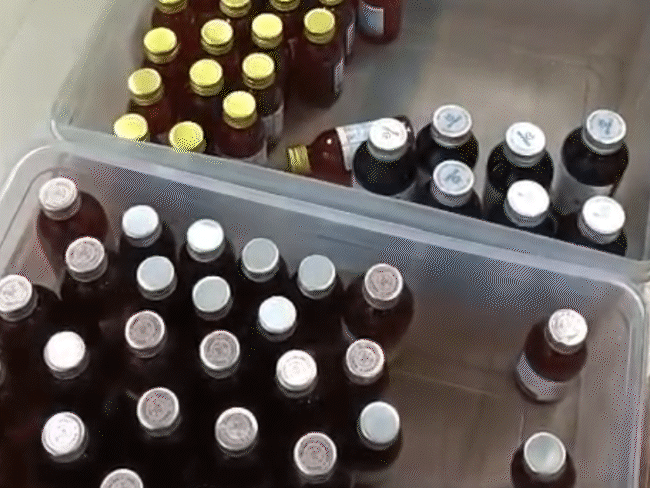
After the death of 25 children due to poisonous cough syrup Coldrif in Chhindwara and Betul of Madhya Pradesh, its impact is now visible on the drug policy of the entire country.
,
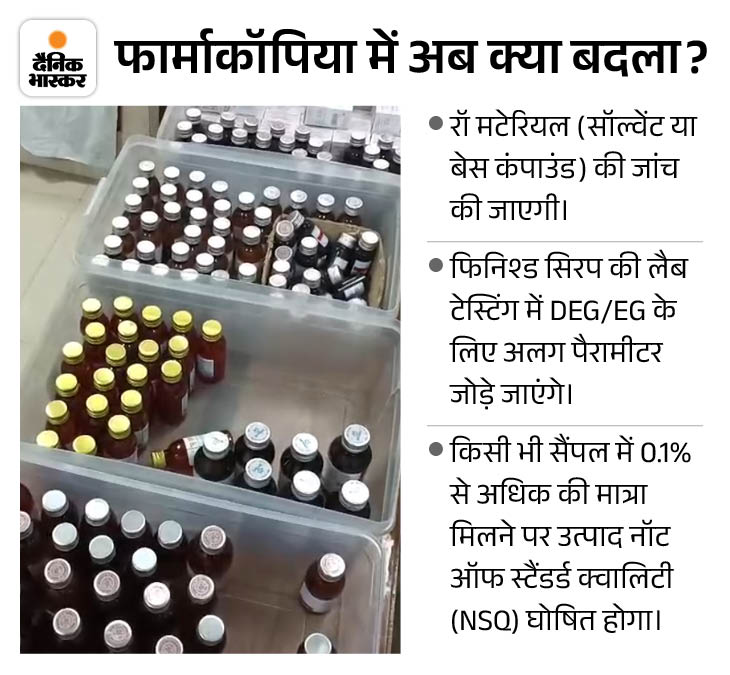
After this incident, now testing of Diethylene Glycol (DEG) and Ethylene Glycol (EG) will be mandatory in every oral liquid syrup manufactured across the country.
Any sample found to have more than 0.1% DEG or EG will be declared “Not a Standard Quality (NSQ)”.
On the basis of the report sent by MP Drug Controller Dinesh Srivastava to Delhi, the Central Government has amended the drug standard book of India – Indian Pharmacopoeia.
Chhindwara report reached Delhi On October 9, 2025, Madhya Pradesh Drug Controller Dinesh Srivastava had sent a detailed report to the Drug Controller General of India (DCGI). Citing the investigation report of Chhindwara Lab, it was said that 46.28% diethylene glycol was found in Coldrif syrup (batch number SR-13), which is toxic to lethal levels.
The report noted that testing for DEGs and EGs is not mandatory in current pharmacopoeia standards, so it should be included in all oral liquid preparations. It was clearly written in the letter that DEG test was not a part of the finished product specification. This was the biggest flaw of this tragedy.

The edition of Indian Pharmacopoeia (EOIP) 2022 was amended.
Central government took action within 24 hours Within 24 hours of receiving the report from the state, i.e. on October 10, 2025, the Drugs Controller General (DCGI) took cognizance of it. After this, the Indian Pharmacopoeia Commission (IPC) took immediate action and issued amendments in the 9th Edition of Indian Pharmacopoeia (EOIP-2022). As soon as this amendment came into effect, it was sent to drug controllers, lab directors, pharma associations and pharmaceutical industry organizations across the country.

The amendment said-

“Based on scientific suggestions, immediate changes are being made in the monographs of some drugs. Now testing of DEGs and EGs will be mandatory in both raw materials and finished products.”



Also read these news related to syrup case…
Attempt to attack poisonous syrup company owner in Parasia Court of Chhindwara

Govindan Ranganathan, the owner of the cough syrup manufacturing company which caused the death of 25 children in Madhya Pradesh, was produced in the Parasia Court of Chhindwara district on Friday. During this time an attempt was made to attack him. People and lawyers raised slogans of ‘hang the murderer’. The court has handed him over on remand for 10 days. Read full news
Chhindwara Collector-SDM knew deaths were happening: but took 15 days to ban the syrup

The deaths of children due to Coldriff cough syrup in Chhindwara could have been avoided if the district administration had taken the alert received from Nagpur seriously. In fact, Dr. Rajesh Aggarwal, director of Colors Hospital, Nagpur, had told this to Parasia’s local councilor Anuj Patkar on September 16 itself. He had also told the Collector-SDM. Read full news
Tamil Nadu company produces Coldrif without testing, quantity of ‘poison’ 486 times more In Madhya Pradesh, so far 25 children have died due to cough syrup in Chhindwara, Betul, Nagpur and Pandhurna. Meanwhile, a big revelation has come to light in the investigation of Coldrif syrup. The report of Tamil Nadu Director of Drugs Control has revealed that this syrup was prepared from non-pharmaceutical grade chemicals. Lab testing also found that the presence of toxic chemicals like diethylene glycol (DEG) and ethylene glycol (EG) in the syrup was 486 times more than the prescribed limit. Read full news